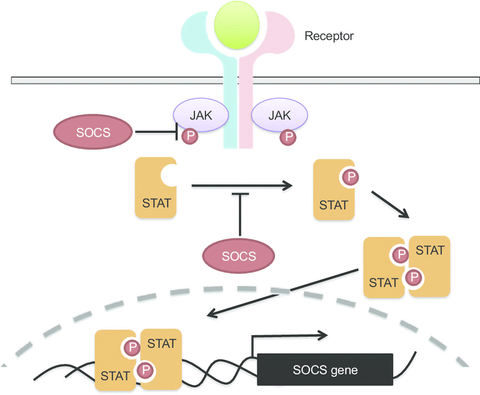
Janus Kinase Inhibitors (JAK inhibitor) interfere with JAK/STAT signaling pathways by cytokine activity inhibition. Although there is overlap, each JAK inhibitor has unique cytokine targets. Historically JAK inhibitors have been used in conditions such as rheumatoid arthritis, psoriatic arthritis, myelofibrosis or polycythemia vera. JAK inhibitors have promising therapeutic potential in COVID-19 due to both their anti-inflammatory and anti-viral effects. Currently, the JAK inhibitor Baricitinib has been found to be an approved treatment option for severe COVID-19 infections. JAK inhibitors have been proposed as a treatment option for COVID 19 because they can decrease the degree of immune activation and overall inflammation. In addition, there is postulation that some JAK inhibitors, specifically Baricitinib, can impact viral infectivity and replication.
At the present time, the combination of Remdesivir and Dexamethasone is considered standard of care. The recommendation for the use of Baricitinib as a therapeutic agent is highlighted by both the research outlined below as well as approval via the FDA. ACTT-4 is currently investigating the use of the combination Remdesivir and Dexamethasone vs. Remdesivir and Baricitinib. Until results from ACTT-4 and future RCTs evaluating the clinical efficacy of Baricitinib are published, we recommend that Baracitinib be used only when the use of steroids are contraindicated.
- The use of Baricitinib in combination with Remdesivir can be considered in the treatment of hospitalized patients with severe COVID-19 (patients requiring supplemental oxygen, high flow oxygen, and non invasive ventilation).
- The benefit of Baricitinib in combination with Remdesivir in patients not on supplemental oxygen or mechanically ventilated is uncertain given the lack of benefit in this group in ACTT-2.
- The use of Baricitinib alone is not recommended.
- The use of Baricitinib in combination with Remdesivir and Dexamethasone (or another equivalent steroid) is not recommended outside of a clinical trial.
Clinical Circumstances
What severity of COVID-19 would you recommend use of this medication (i.e. mild, moderate, or severe illness; outpatient vs inpatient use)?
- Moderate to severe cases as defined as patients requiring supplemental oxygen, high flow oxygen, and non-invasive mechanical ventilation.
Would you recommend restricting this medication in some way (for example, “can be considered in consultation with ID”)?
- Consultation with Infectious Diseases and Rheumatology
Medication-specific Consideration
Based on the current literature, what dosing is recommended?
- 4 mg daily for 10 days of the JAK inhibitor in question. JAK dosing is specific to each JAK and should not be extrapolated from that of Baritcitinib. If use of other JAK inhibitor is considered, providers should work with their pharmacist and reference the literature review below for dosages.
Is drug monitoring required? If so, what is suggested?
- While on therapy monitor CBC w/ diff, basic metabolic panel, liver function tests
- JAK inhibitors can demonstrate dose dependent side effects including myelosuppression, transaminitis, risk for viral (Herpes zoster virus and/or Hepatitis B) or tuberculosis reactivation, and risk for thrombosis. The adverse events associated with JAK inhibitors are rare and the majority of the studies evaluating adverse events have involved chronic use of these agents.
- JAK inhibitors can be metabolized by CYP3 system in the liver so recommend evaluating for medication interactions
Supply and procurement consideration: Is treatment currently available for use in our system, and if so are shortages anticipated? Are there currently restrictions or other barriers?
- JAK inhibitors are available for use in the UM Health Fairview System. Nationwide shortages are possible due to limited hospitals having access to JAK inhibitors as well as it being an outpatient medication leaving hospitals with little to no drug on hand.
Use in special populations. Please consider use in special populations (pregnancy, immunosuppressed, kidney or liver disease, etc.) and outline any concerns below.
- The only approved use in pregnancy if the potential benefit outweighs the risk of mother and fetus. Embryo-fetal toxicities including skeletal anomalies and reduced fertility have been observed in animals dosed in excess of maximum human exposure.
- The data is not sufficient enough to inform the risks for birth defects or miscarriage after baricitinib use. The only approved use in pediatrics is ≥ 9 years of age with 4 mg once daily for 14 days of baricitinib in case of emergency. Not recommended for children ages 2-8 years old.
- Baricitinib is not recommended for patients who have acute kidney injury or ESRD requiring dialysis.
- Baricitinib has not been studied in patients with severe hepatic impairment and should only be used if the benefit outweighs the risks. It is unknown if dose adjustment is needed in patients with hepatic impairment receiving Baricitinib.
 = Supporting use article = Supporting use article |
 = Neutral Article = Neutral Article |
 = Contradicting use article = Contradicting use article |

 |
 |
 |
        |
     |
 |
Peer-reviewed Studies
 Randomized Controlled Trial
Randomized Controlled Trial
 Cao et al 2020 “Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial” J Allergy Clin Immunol.
Cao et al 2020 “Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial” J Allergy Clin Immunol.
- PROSPECTIVE RCT, multicenter, single-blind, phase II trial
- 1:1 assignment of 41 patients
- inclusion = COVID19+, 18yo+, <75yo, severe cases
- exclusion= concomitant malignant tumors, severe uncontrolled cardiovascular +/- metabolic disease, mental/severe psychiatric disorder, need for invasive vent at time of recruitment, patients who could not followup, pregnant or lactating women, other active infections.
- Treatment = 5 mg twice per day for ?10 days (not specified!) + SOC versus placebo + SOC. Corticosteroid and antiviral use was balanced between two groups.
- Endpoints = 1° is time to clinical improvement. 2° includes improvement rate, recovery of lymphopenia, time to invasive ventilation, duration of hospitalization for survivors, time to death, time to viral clearance, serious adverse events at 28 days, viral clearance, anti-SARS-CoV2 IgG and IgM antibodies, overall mortality
- Results = 1° endpoint no statistical or numeric difference, in regards to secondary endpoints most were not significantly or numerically different except for 28 day mortality (14% in control, 0% in ruxolitinib group) and recovery of lymphopenia (median 5 days in ruxolitinib versus 8 days in control). Importantly, no difference in days to discharge, viral clearance, adverse events. Several cytokines/chemokines were reduced in ruxolitinib group.
- Caveats = well designed study hindered by small sample size due to limited recruitment towards end of pandemic.
- Overall = neutral study.
-
 Kalil et al March 4, 2021. “Baricitinib plus Remdesivir for Hospitalized adults with Covid-19.” The New England Journal of Medicine.
Kalil et al March 4, 2021. “Baricitinib plus Remdesivir for Hospitalized adults with Covid-19.” The New England Journal of Medicine.
- Randomized, double-blind, placebo-controlled trial
N= 1033, 515 Remdesivir and Baracitnib and 518 Remdesivir and placebo
Inclusion=oxygen saturations ≤ 94% on RA, requiring supplemental O2, mechanical ventilation or ECMO, presence of radiographic infiltrates and COVID-19 PCR positive.
Exclusion: LFT elevation > 5x the upper limit of normal, expected discharge in 72 hours, impaired renal function, requiring dialysis, breastfeeding/pregnancy, allergy
Treatment = 4 mg daily dose of Baricitinib vs placebo plus 200 mg remdesivir loading dose followed by 100 mg maintenance dose.
Results = median time to recovery of 7 days compared to 8 days with control (P value=0.03). Those who received combination therapy had improvement in their clinical status based on an ordinal scale at day 15. Median time to recovery among patients receiving noninvasive ventilation or high-flow oxygen was 10 days in the combination group and 18 days in the control group. Serious adverse events and risk for new infections was less in the combination group.
Caveats = Inclusion of minority participants. There were decreased mortality rates amongst the combination group but this study was not powered to completely evaluate mortality.
Overall = positive; remdesivir + baricitinib is potentially an effective treatment combination for SAR-CoV-2. The benefit of combination therapy was greatest in ordinal scale 5 (supplemental oxygen) and ordinal scale 6 (high flow oxygen or noninvasive ventilation). The benefit was not as clear in ordinal scale 4 (no oxygen) or ordinal scale 7 (mechanical ventilation).
- Randomized, double-blind, placebo-controlled trial
 Giudice et all Jun 2020. “Combination of Ruxolitinib and Eculizumab for Treatment of Severe SARS-CoV-2-Related Acute Respiratory Distress Syndrome: A Controlled Study.” Frontiers in Pharmacology.
Giudice et all Jun 2020. “Combination of Ruxolitinib and Eculizumab for Treatment of Severe SARS-CoV-2-Related Acute Respiratory Distress Syndrome: A Controlled Study.” Frontiers in Pharmacology.
- Randomized placebo -controlled trial
N = 17; 7 JAK inhibitor treatment and 10 standardize treatment only (heparin, hydroxychloroquine and or mechanical ventilation)
Inclusion = moderate to severe ARDS
Exclusion = age 17 or younger, negative for SARS-CoV-2 infection, diagnosis of mild COVID-19, active infections including TB or other clinical conditions that contraindicate the use other these JAK inhibitors, hypertransaminasemia and or cytopenia, pregnant or breastfeeding women.
Treatment = treatment with ruxolitinib and eculizumab: at day 0, ruxolitinib, 10 mg/twice daily and eculizumab 900 mg IV; ruxolitinib was given twice daily every day for 14 days; while, eculizumab was administered at day 7 and, when needed, at day 14 for a total maximum dose of three. Antibiotic prophylaxis with azithromycin was started in all 17 subjects. Steroids at 20 mg/twice daily were administered in five out of seven subjects in the treated group, and in three out of 10 in BAT group.
Results = Treatment group showed clinical improvement after 3 days of treatment, showed a higher PaO2 and PaO2/ FiO2 ratio and a decreased in circulating D-dimer levels and an increase in platelet count. No patient on treatment required invasive mechanical ventilation or high flow nasal oxygenation after or during treatment.
Caveats = No difference between the two groups observed in lactate dehydrogenase levels, hemoglobin levels, and WBC counts. Risk of meningococcal infection is increased only after several weeks of eculizumab. Well designed study hindered by small sample size, needing a larger trial to confirm data. RCT brings validity as gold standard of study.
Overall = positive potential as a treatment for COVID-19
- Randomized placebo -controlled trial
 Salma et al Dec 2020. “Tocilizumab in patients hospitalized with covid-19 pneumonia.” The New England Journal of Medicine.
Salma et al Dec 2020. “Tocilizumab in patients hospitalized with covid-19 pneumonia.” The New England Journal of Medicine.
- Randomized, double-blind, placebo-controlled, phase 3 trial
- N=389
- Inclusion = sites enrolling high-risk and minority populations, patients over age 18 with no upper age limit hospitalized with COVID-19.
- Exclusion = if patients were receiving continuous positive airway pressure, bilevel positive airway pressure, or mechanical ventilation. If progression of illness to death was imminent and inevitable within 24 hours or if they had active tuberculosis or suspected active bacterial, fungal, or viral infection other than COVID-19.
- Treatment = 8 mg per kilogram of body weight, to a maximum of 800 mg per dose in addition to standard of care (antiviral treatment, limited systemic glucocorticoids <1mg p kg of body weight, and supportive care).
- Results = The cumulative percentage of patients who had received mechanical ventilation or who had died by day 28 was significantly lower in the tocilizumab group than in the placebo group. The median time to hospital discharge or readiness for discharge over the 28-day period was 6.0 days in the tocilizumab group and 7.5 days in the placebo group. The results were similar when death was treated as a competing risk. The median time to improvement in clinical status as assessed with the seven-category ordinal scale over the 28-day period was 6.0 days with tocilizumab and 7.0 days with placebo. The results were similar when death was treated as a competing risk.The median time to clinical failure over the 28-day period could not be estimated in either group. Mortality by day 28 was 10.4% in the tocilizumab group and 8.6% in the placebo group.
- Caveats = I commend the study on its inclusion of minority populations. 2:1 randomization shows complex statistical concerns which are harder for non-experts to assess the statistical validity of the design. RCT brings a lot of validity to the study’s statements.
- Overall = neutral
 Observational Studies
Observational Studies
 Cantini et al 2020 “Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact” Journal of Infection.
Cantini et al 2020 “Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact” Journal of Infection.
- OBSERVATIONAL, CASE SERIES, HISTORICAL COHORT MATCHED
- Case n = 12; cohort n = 12
- inclusion = moderate COVID19, 18yo+, already on HCQ or antiviral
- exclusion= history of thrombophlebitis, latent TB, pregnancy, lactation
- cohort = previous patients treated with HCQ or antiviral
- Treatment = 4 mg per day for 2 weeks
- Results = improvement in clinical (fever, oxygenation, ICU transfer, etc) and laboratory markers at 1 week (CRP, etc). Improvements sustained at 2 weeks, many discharged home. No adverse events noted in treatment group.
- Caveats = treatment group started with higher CRP, better blood pressure. One patient discontinued baricitinib early due to LFT elevation though authors attribute that to antiviral. Distribution of antiviral/HCQ use is not reported. No long term followup reported. Use of antibiotics not reported.
 Rosée et al 2020 “The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation” Leukemia.
Rosée et al 2020 “The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation” Leukemia.
- OBSERVATIONAL, RETROSPECtiVE CASE SERIES
- Case n = 14
- inclusion = COVID19+, clinical inflammation score >10
- exclusion= none
- Treatment = 7.5 mg BID uptitrated to 15 mg BID
- Results = improvement in clinical inflammation score, regardless of steroid use, in 12/14 within 7 days of ruxolitinib induction. 11/14 with sustained improvement. No red flags.
- Caveats = laboratory based outcome, not clinically, no matched comparative cohort to indicate if any significant change in clinical outcomes.
- Overall = unclear
 Stebbing et al 2020 “Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients.” EMBO Molecular Medicine.
Stebbing et al 2020 “Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients.” EMBO Molecular Medicine.
- Type of study: Clinical case series phase 2b randomized placebo controlled study and biochemical and cellular evidence
- n = baricitinib 1 mg (n = 23), 2 mg (n = 24), 4 mg (n = 23), and 8 mg (n = 23)
- Inclusion: patients with signs of severe illness at diagnosis or secondary clinical aggravation such as respiratory symptoms or general signs based on World Health Organization criteria for severe pneumonia caused by SARS‐CoV‐2.
- Exclusion: none listed
- Treatment = 2 mg, 4mg, and 8 mg once daily doses
- Results = Inhibition of cytokines implicated in COVID-19 infection (IL2, IL6, IL10, IFN gamma, and HCSF) with lower half-max inhibitory concentration values. Significant reduction in baseline of plasma IL6 at week 12 in patients with active RA in randomized placebo controlled dose ranging study. High affinity against AAK1, BIKE, STK16, and GAK which facilitates viral propagation of coronavirus in epithelial cells. Reduced viral infectivity in 3D primary human liver spheroids infected with SARS-CoV-2.
- Caveats = Performed via AI; laboratory based outcome. Case series design is subject to selection bias and does not have a control arm – findings may not be generalizable to a larger population.
 D’Alessio et al Nov 2020. “Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia.” Leukemia.
D’Alessio et al Nov 2020. “Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia.” Leukemia.
- Non-randomized case series
- N= 75; 18 excluded from analysis due to not meeting criteria
- Inclusion= a nasopharyngeal swab positive for SARS-CoV2 by polymerase chain reaction, pulmonary infiltrates typical for interstitial pneumonia on Chest CT and respiratory frequency >30/min or oxygen saturation equal or lower than 93%, over 18 years old.
- Exclusion = Patients with chronic comorbidities or neoplastic disease with <1 year life expectancy, documented bacterial superinfections, advanced dementia, previous treatment with anti-interleukin 1 or anti-interleukin 6 inhibitors.
Treatment = Ruxolitinib was administered orally at a dose of 5 mg twice daily for 7 days and then tapered to 5 mg daily to complete a 10-day course of treatment. In addition to ruxolitinib all patients received methyl-prednisolone 1 mg/kg intravenously for 3 days followed by 0.5 mg/kg for 5 days and then oral prednisone, which was slowly tapered in the course of 2 weeks. Concomitant antiviral therapy such as hydroxychloroquine, lopinavir/ritonavir, remdesivir was not permitted during treatment with ruxolitinib. - Results = Twenty-four patients of group A (75%) were considered clinically recovered without admission to the ICU, five (16%) were transferred to the ICU, mechanically ventilated and clinically cured and three patients (9%) died, one of whom in the ICU. Ruxolitinib was withdrawn in all five patients admitted to the ICU, while steroid was continued as initially planned. No rebound of inflammation was observed in these patients. On the contrary only 27 patients of group B (63%) were considered clinically recovered, three (7%) were transferred to the ICU and clinically cured and 13 died (30%), three of whom in the ICU. Found a significant reduction in mortality and no significant adverse event in treated patients compared to controls. Faster decline in CRP levels and disappearance of fever in treated patients.
- Caveats = Case series study: selection bias, multiple treatment effects, post intervention follow-up period, no comparison arm. Non- randomization leaves the study susceptible to selection bias.
- Overall = neutral
 Rodriguez-Garcia, et al Oct 2020. “Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study.”
Rodriguez-Garcia, et al Oct 2020. “Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study.”
- Observational cohort study
- N= 62 (low dose n=40 and high dose n=22). A total of 112 patients were analyzed: corticosteroids group (CS, n = 50) and baricitinib and corticosteroids group (BCT-CS, n = 62).
- Inclusion= older than 18 years admitted to the hospital because of SARS-CoV-2 pneumonia who had an arterial oxygen partial pressure (PaO2)/fractional inspired oxygen (FiO2) ratio <200 mmHg on the hospital ward (moderate to severe SARS-CoV-2 pneumonia).
- Exclusion = Patients were excluded if they had major comorbidities (chronic heart failure, chronic obstructive pulmonary disease on oxygen therapy, obstructive sleep apnea syndrome with continuous positive airway pressure, advanced chronic kidney disease, active malignancies). had previously been treated with other immunomodulators (IVIG, INF, anakinra, tocilizumab). Patients admitted to ICU or who died were considered non-evaluable and thus excluded.
- Treatment = In the baricitinib plus corticosteroids group (BCT-CS, n = 62) patients received corticosteroids for 3 days and then prednisone, combined with baricitinib for 5–10 days. Doses of corticosteroids included 6-methylprednisolone 80, 125 or 250 mg/day. Baricitinib was administered under two schemes: a loading dose of 4 mg the first day and then 2 mg daily (low-dose baricitinib, n = 40) or 4 mg daily each day (high-dose baricitinib, n = 22). Patients older than 75 years received low-dose baricitinib.
- Results = At baseline, patients receiving BCT-CS high dose differed from patients receiving BCT-CS low dose with respect to diastolic blood pressure, ferritin, lymphocyte count, high-flow oxygen and non-invasive ventilation, and methylprednisolone total dose. Patients on the high dose had also lower SpO2/FiO2 on ward. After enrolment, patients on high-dose BCT-CS needed more intensive ventilatory support (high-flow oxygen, non-invasive ventilation) and received a higher dose of methylprednisolone compared with patients on low-dose BCT-CS.
- Caveats = Well designed study hindered by small sample size. Hard to determine a cause and effect relationship from descriptive research such as an observational study. Cohort study has a lack of so its external validity is lower than that of study designs where the researcher randomly assigns participant.
- Overall = neutral
 Stebbing et al Nov 2020. “JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality.” Science Advances.
Stebbing et al Nov 2020. “JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality.” Science Advances.
- Cohort study
- N = 83 with a total of 166 patients analyzed
- Inclusion = Patients with radiologically defined COVID-19 pneumonia and laboratory confirmed infection, as diagnosed by a positive SARS-CoV-2 RT-PCR test by nasopharyngeal swab. All patients enrolled had an SaO2 < 94% at baseline but did not require mechanical ventilation.
- Exclusion = history of active or latent tuberculosis infection (QuantiFERON Plus-test positivity, Qiagen, Germany), pregnancy and/or lactation. Treatment 4 mg/day for 14 days in conjunction with standard of care in Italy, and at lower doses of 2 or 4 mg/day for 3 to 11 days in the Spanish cohort because of age-related factors
- Results = Reversed IFN-induced gene expression changes. Genes that were highly induced upon IFN treatment were significantly down-regulated (including the IFN-stimulated genes (ISG) 15 and 20, chemokines, MHC components, and several members of the interferon induced protein family). Reverses the IFN-mediated inhibition of immunoreceptor signaling, metabolic remodelers and transcriptional regulators. Well tolerated with a reduction in inflammation and substantially improved outcomes. Effects were evident from the first treatment days and maintained over follow-up.
- Caveats = Cohort study has a lack of so its external validity is lower than that of study designs where the researcher randomly assigns participant. Unrandomized trial so unknown confounding variables could have compromised the results (ex existing comorbidities). Small sample size limits statistical power. Multiple statistical tests were performed without an adjustment for multiplicity, which increases the risk of a type-I statistical error.
- Overall = neutral
 Bronte et all Dec 2020. “Baricitinib restrains the immune dysregulation in patients with severe COVID-19.” The Journal of Clinical Investigation.
Bronte et all Dec 2020. “Baricitinib restrains the immune dysregulation in patients with severe COVID-19.” The Journal of Clinical Investigation.
- Cohort study
- N = 20; 56 controls
- Inclusion = clinical onset of symptoms not exceeding 9 days and the presence of interstitial lung involvement not exceeding 50% on chest x-ray or CT were required for patients to receive baricitinib therapy.
- Exclusion = the presence of active malignancies and/or immunodeficiency, cardiovascular disease with recent myocardial infarction or stroke, as well as thrombophilia or deep venous thrombosis or pulmonary thromboembolism. Additional exclusion criteria included the presence of chronic kidney disease with renal failure, cirrhosis with a Child-Pugh score of C, or the presence of anemia or severe neutropenia or lymphocytopenia
- Treatment = 4 mg baricitinib twice daily for 2 days followed by 4 mg per day for the remaining 7 days. A low dose of 2 mg twice daily for 2 days followed by 2 mg per day was maintained for patients older than 75 years.
- Results = Improving the clinical parameters of SARS-CoV-2 infection. Faster reduction in the need for oxygen flow therapy (P < 0.001) and a more rapid increase in the P/F ratio compared with the control group (P = 0.02), as well as a reduction in serum levels of C-reactive protein (CRP)
- Caveats = Study observed no significant difference in ARDS incidence or disease duration, expressed as the number of hospitalization days. Small sample size. Hard to determine a cause and effect relationship from descriptive research such as an observational study. This off-label program self reports some missing data for several outcomes such as immunological parameters for some of the enrolled patients. Study was not double-blinded. Cohort study has a lack of so its external validity is lower than that of study designs where the researcher randomly assigns participant.
- Overall = neutral
Major peer-reviewed studies providing context for therapy
- Gavegnanno et al. 2019. “Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro.” Journal of Neuroinflammation.
- PRECLINICAL, NOT SARS1 or 2 RELATED
- Sanchez et al. 2018. “JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies.” J Clin Invest.
- CLINICAL, CASE SERIES, NOT SARS1 or 2 RELATED
- Ahmed et al. 2019. “Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial.” Lancet Hematology.
- CLINICAL, CASE SERIES, NOT SARS1 or 2 RELATED but includes EBV associated HLH
List of pre-peer reviewed/pre-publication studies
- Hoang et all Sept 2020. “Barcitinib treatment resolves lower airway inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques.” bioRxiv.
Randomized Non-human primate (NHP) models
N = 8 adult rhesus monkeys.
Treatment = 4 mg of oral baricitinib, daily for 8–9 days.
Results = Baricitinib treated RMs displayed reduced (i) lung pathology, from moderate in untreated animals to mild; (ii) levels of inflammatory cytokines, chemokines, and signaling pathways associated with inflammation, neutrophil recruitment, and disease progression in SARS-CoV-2 infected humans; and (iii) levels of systemic inflammation that are associated with COVID-19 severity in humans while not having an impact on Type 1 IFN responses. Reduced infiltration of neutrophils into the lungs, and a highly reduced T cell activation in both blood and BAL as compared to untreated animals. Furthermore, in this study we were able to observe an increased NETosis activity of neutrophils upon SARS-CoV-2 infection, previously described in serum from COVID19 patients.
Caveats = Though well-tolerated, Baricitinib did not limit viral replication of SARS-CoV-2 infected RMs.
Overall = neutral
JAK inhibitors: background, potential pathways related to COVID-19, current state of clinical data
Inhibitors of JAK/STAT pathways that are signaling mechanism for many cytokines. (see Figure 1). They are small molecule inhibitors, pill form only, theoretically easier to increase mass production compared to biologics. There are several in development, more in pre-clinical/animal trials, and some that already have approvals for multiple indications. I have listed all the ones that already have FDA approval and are used more frequently in table 1. When reviewing JAK specificity IC50s, keep in mind that relative binding for one drug is more important than best IC50 across multiple drugs as actual signal inhibition also depends on dose of drug used—larger within drug JAK specificity simply widens the therapeutic window. JAK inhibitors do have varying level of promiscuity for different JAKs and this is further complicated by each individual JAK being involved in more than one cytokine pathway. In general, most JAK inhibitors demonstrated dose dependent side effects of variable degrees of myelosuppression, increase risk of viral recurrence (particularly Herpes zoster virus reactivation (shingles) and Hepatitis B), and increased risk for thrombosis. Risk of thrombosis and infection is likely dose and duration dependent, however myelosuppression can occur within a week.
| JAK inhibitor | Current uses | JAK specificity | Signaling affected | Side effects | Other |
|---|---|---|---|---|---|
|
Ruxolitinib (Jakafi)
Novartis & Incyte Pharmaceuticals |
FDA approved indications: GvHD Myelofibrosis Polycythemia vera (JAK2 mutation)
Off-label use: Secondary HLH (aka MAS) prelim/ongoing trials at UMichigan |
JAK1=JAK2>TYK2>>JAK3
IC50s (nM +/- SD) (fold from highest IC50) JAK1 3.3 +/- 1.2 (1x) JAK2 2.8 +/- 1.2 (1x) JAK3 428 +/- 243 (140x) TYK2 19 +/- 3.2 (6x)
life: 3-6 hours (including metabolites) |
Inhibits:
Might inhibit:
Will not directly inhibit:
|
>10%: anemia, thrombocytopenia, increased LFTs, neutropenia, dizziness, headaches, pruritis, hypertriglyceridemia, muscle spasms, dyspnea
<10% of interest: Herpes Zoster, arthralgia, apistaxis, nasopharyngitis, cough
Postmarketing: exacerbation of hepatitis B, PML, TB |
CYP3A4 processing, avoid grapefruit juice
Pregnancy: adverse event observed in animal reproductive studies
Lactation: unknown |
|
Bariticinib (Olumiant) |
FDA approved indications: Rheumatoid Arthritis
Off-label use: |
JAK1=JAK2>>TYK2>>JAK3
IC50s (nM +/- SD) ) (fold from highest IC50) JAK1 5.9 +/- 0.9 (1x) JAK2 5.7 +/- 1.7 (1x) JAK3 >400 (>66x) TYK2 53 (8x)
life: 12 hr |
Same as ruxolitinib |
>10%: URTI
<10%: nausea, herpes zoster, increased LFTs
Postmarketing: arterial thrombosis, BK virus, CMV viremia, GI perf, herpes virus infection, histoplasmosis, mycobacterium infection, opportunistic infection, PJP, PE, TB, venous thrombosis
BLACK BOX: infections, DVT/PE/arterial thrombosis |
BCRP/ABCG2, CYP3A4 processing
Pregnancy: adverse events observed in animal studies
Lactation: unknown |
|
Tofacitinib (Xeljanz) |
FDA approved indications: Psoriatic arthritis (5 mg BID) RA (5 mg BID) UC (10 mg BID) Extended release formulation available
Off-label use: |
JAK3>JAK1=JAK2>TYK2
IC50s (nM +/- SD) ) (fold from highest IC50) JAK1 3.2 +/- 1.4 (2x) JAK2 4.1 +/- 0.4 (3x) JAK3 1.6 +/- 0.2 (1x) TYK2 34 +/- 6 (28x)
life: 3 hours (IR)
Absorption decrease with high fat meal but AUC unchanged |
Inhibits:
Less likely to inhibit
Will not directly inhibit:
|
>10%: infection, nasopharyngitis
<10%: headache, skin rash, increased serum cholesterol, diarrhea, gastroenteritis, nausea, UTI, anemia, herpes zoster (can be severe), increased CK
Postmarketing: angioedema, arterial thrombosis, BK virus, CMV, DVT, candidiasis, GI perf, hepatotoxicity, histo, listeriosis, lymphocytopenia, mycobacterium infection, neutropenia, PE, reactivation fo HBV, TB, urticaria, TB
BLACK BOX: infections, thrombosis DVT/PE/arterial, sudden cardiac death at higher doses |
CYP3A4 & CYP2C19 processing
Pregnancy: limited data
Lactation: unknown |
|
Upacitinib (Rinvoq)
Abbvie |
FDA approved indications: RA (15 mg QD)
Off-label use: |
JAK1=JAK2>>>JAK3>TYK2
IC50s (nM +/- SD) ) (fold from highest IC50) JAK1 47 +/- 6.1 (1x) JAK2 120 +/- 29.6 (2.5x) JAK3 2304 +/- 380 (50x) TYK2 4690 +/- (100x)
life = 2-4 hr |
Same as ruxolitinib
Except less likely to affect JAK3 and TYK2 mediated signaling:
|
>10%: URTI
<10%: nausea, neutropenia, increased LFTs, increased CPK, cough, fever, herpes simplex, herpes zoster, oral candidiasis, pneumonia, TB, fungals, opportunistic, HBV reactivation
BLACK BOX: infections, thrombosis |
CYP2D6, CYP3A4 processing
Pregnancy:
Lactation:
|
|
Fedratinib |
FDA approved indications: Myelofibrosis |
JAK2>>JAK1>TYK2>TYK2
IC50s (nM +/- SD) (fold from highest IC50) JAK1 105 (35x) JAK2 3 (1x) JAK3 1002 (334x) TYK2 405 (135x)
life = 41 hours |
Inhibits:
Might Inhibit:
Unlikely to inhibit:
Will not directly inhibit:
|
>10%: fatigue, hyponatremia, increased amylase/lipase, n/v/d, anemia, thrombocytopenia, neutropenia, increased LFTs, AKI
<10%: heart failure, cardiogenic shock, headache, dizziness, UTI, severe anemia, limb pain, ostealgia, encephalopathy (like wenickes), GI toxicity, cystitis
BLACK BOX: encephalopathy can be fatal |
CYP3A4, CYO2C19 processing
Pregnancy: adverse events observed in animal studies
Lactation: unknown |
JAK inhibitors: theories of utility for COVID19
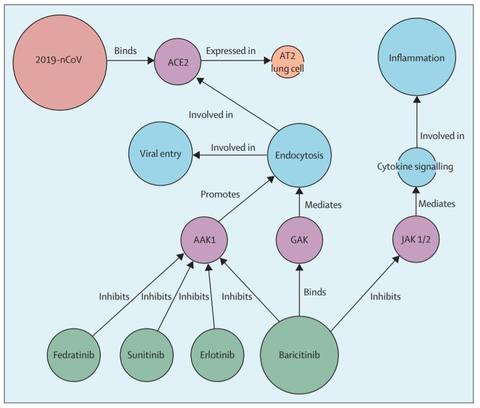
Baricitinib specific antiviral activity is hypothesized by artificial intelligence algorithms to arise from inhibition of the numb-associated kinase (NAK) AAK1 which is an important regulator of clathrin-mediated endocytosis. Inhibition of AAK1 would likely reduce the ability of viruses to infect lung cells, and is being proposed as a pharmacological mechanism that warrants further investigation as a treatment for SARS-CoV-2 infection (COVID-19). (Stebbing et al 2020 “COVID19: combining antiviral and anti-inflammatory treatments. Lancet. Feb 27, 2020)
In addition, the powerful anti-inflammatory activity of JAK inhibitors in general is proposed to be effective against the pathological effects of raised cytokine levels (e.g. interferon γ, IL-6, etc) in patients with hyper-inflammation syndrome such as cytokine release syndrome, HLH, secondary HLH aka MAS. (Favalli et al. Autoimmune Rev. March 20, 2020) This has been demonstrated in case series publications for ruxolitinib in secondary HLH (Ahmed et al. 2019. “Ruxolitinib in adult patients with secondary hemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial” Sept 16, 2019. Lancet Hematology.)
In regards to Baricitinib, it has been shown in modified murine models to reverse cognitive deficits and curtail inflammatory markers in HIV-associated neurocognitive disorders (HAND). (Gavegnano et al. Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro. September 2019. Journal of Neuroinflammation. doi: 10.1186/s12974-019-1565-6). A small study of 18 patients, comprising of 10 patients with CANDLE (chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures), 4 patients with SAVI (stimulator of IFN genes-associated [STING-associated] vasculopathy with onset in infancy), and 4 patients with other interferonopathies received Baricitinib treatment in an expanded access program. Clinical manifestations and inflammatory and IFN biomarkers improved in patients with the monogenic interferonopathies. One CANDLE patient discontinued treatment because of BK viremia and azotemia (Sanchez et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest 2018; 128.)
Considerations proposed for study associated use
- If patients meet HLH criteria, first line therapy is IL-1 inhibition and high dose steroids. However, JAK1/2 inhibitors, as demonstrated by ruxolitinib and baricitinib case series are efficacious in these cases as well
- note that absorption in critical patient may be an issue as these medications are ONLY orally available
- anticipate some laboratory response within 1 week, consider discontinuation if not response
- anticipate some side effects within 1 week (specifically, myelosuppression)
- in patients with high risk for thrombosis at baseline, consider anticoagulation if adding on JAK inhibitor
- monitor closely for secondary infections. Patients may not be able to mount a fever. Consider every other day montoring blood cultures
- Careful review of current medication to screen for cytochrome incompatibility!! Consider dose reduction.
- Special populations:
- JAK inhibitors are not safe in pregnancy, lactation
- JAK inhibitors have been used in pediatric HLH
- Immune suppressed patients would need their immune suppression held while receiving JAK inhibitors
Current clinical trials
Notable trials
- Strategy of chemoprophylaxis with HCQ against COVID-19 infection in patients diagnosed with an immunomediated inflammatory disease who are following a treatment with biological agents and / or Jak inhibitors.
- Phase 2 study of inhaled pan-Janus kinase inhibitor TD-0903 in treatment of acute lung injury associated with COVID-19
Baricitinib trials:
- Treatment of COVID-19 in hospitalized patients with Baricitinib
- Safety and efficacy for Baricitinib for COVID-19
- Baricitinib in symptomatic patients infected by COVID-19
- ACTT4 evaluation of barcitinib and remdesivir as COVID-19 treatment
- A study of Baricitinib (LY3009104) in participants with COVID-19 (COV-BARRIER). To see if the study drug baricitinib is effective in hospitalized participants with COVID-19.
- Baricitinib therapy in COVID-19: Retrospective study on the efficacy of baricitinib in 12 COVID-19 patients with moderate pneumonia
- Baricitinib, placebo, ad antiviral therapy for the treatment of patients with moderate and severe COVID-19. This phase II trial studies the effect of baricitinib in combination with antiviral therapy for the treatment of patients with moderate or severe coronavirus disease-2019 (COVID-19).
- Baricitinib compared to standard therapy in patients with COVID-19 (BARICIVID-19). This is a multicenter randomized clinical trial that aims to evaluate the efficacy and safety of baricitinib in patients with SARS-CoV2 pneumonia. Patients will be randomized to receive or not baricitinib as adjunctive therapy.
- Clinical epidemiological characterization of COVID-19 disease in hospitalized older adults. Retrospective clinical-epidemiological study aimed at characterizing COVID-19 disease in adults older than 70 years, hospitalized in the "Perpetuo Socorro" Hospital of Albacete (Spain) from 09/03/2020 until 20/04/2020.
- Clinical trial to evaluate efficacy of 3 types of treatment in patients with pneumonia by COVID-19 (COVID19COVINIB). The study aims to compare Imatinib 400mg, Baricitinib 4mg or supportive treatment, administered for 7 days in the setting of SARS-CoV-2 pneumonia treatment.
- Multi-arm therapeutic study in pre-ICU patients admitted with COVID-19 – repurposed drugs (TACTIC-R). The medications investigated for efficacy in this trial are Baricitinib and Ravulizumab.
- Adaptive COVID-19 treatment trial 2 (ACTT-2). ACTT-2 will evaluate the combination of baricitinib and remdesivir compared to remdesivir alone. Subjects will be assessed daily while hospitalized.
- Baricitinib for corona virus pneumonia (COVID-19) a therapeutic trial (BREATH).
- Factors associated with clinical outcomes in patients hospitalized for COVID-19 in GHT-93 est. This cohort study aims to assess factors associated with clinical outcomes in patients hospitalized for Covid-19, by analyzing associations between treatments and outcomes.
- Effect of treatments in patients hospitalized for severe COVID-19 pneumonia: a multicenter cohort study.
- Treatment of moderate to severe coronavirus disease (COVID19) in hospitalized patients.
Ruxolitinib trials
- Ruxolitinib for acute respiratory disorder syndrome due to COVID-19
- Assessment of efficacy and safety of Ruxolitinib in patient’s with COVID-19 associated ARDS who require mechanical ventilation
- Safety and efficacy of ruxolitinib for COVID-19
- Expanded access program of ruxolitinib for the emergency treatment of cytokine storm from COVID-19 infection.
- Ruxolitinib in the treatment of COVID-19.
- Phase 3 randomized, double blind, placebo-controlled multi center study to assess the efficacy and safety of ruxolitinib in patients with COVID-19 associated cytokines storm (RUXCOVID).
- Treatment of SARS caused by COVID-19 with ruxolitinib.
- Ruxolitinib managed access program (MAP) for patients diagnosed with severe/ very severe COVID-19 illness.
- Ruxolitinib in covid-19 patients with defined hyperinflammation (RUXCoFlam).
- Ruxolitinib for the treatment of acute respiratory distress syndrome in patients with COVID-19 infection (RESPIRE). It is an observational, cohort, retrospective, monocentric, non-profit study. The primary objective is to evaluate the efficacy and safety of ruxolitinib in acute respiratory distress syndrome in patients with SARS-CoV-2 COVID-19 with rapid deterioration of respiratory parameters in the last 12 hours.
- A trial using ANAKINRA, tocilizumab alone or in association with ruxolitinib in severe stage 2b and 3 of COVID-19 associated disease (INFLAMMAOV). An open randomized therapeutic trial (1/1/1) on 216 patients with severe stage 2b and 3 of the disease
- Therapeutic plasma exchange alone or in combination with ruxolitinib in COVID-19 associated CRS. This protocol will evaluate the efficacy of Therapeutic Plasma Exchange alone or in combination with ruxolitinib in COVID positive patients with PENN grade 2, 3, 4 cytokine release syndrome. It is hypothesized that dual intervention of acute apheretic depletion of cytokines and concomitant suppression of production will produce superior amelioration of the cytokine load and to help to prevent cytokine load rebound.
- Ruxolitinib for treatment of COVID-19 induced lung injury ARDS (RuXoCoil).
- Inflammatory signal inhibitors for COVID-19 (MATIS): The Multi-arm trial of Inflammatory Signal Inhibitors for COVID-19 (MATIS) study is a two-stage, open-label, randomised controlled trial assessing the efficacy of ruxolitinib (RUX) and fostamatinib (FOS) individually, compared to standard of care in the treatment of COVID-19 pneumonia. N = 171 in stage 1 and n = 285 in stage 2.
- Study of Ruxolitinib plus simvistatin in the prevention and treatment of respiratory failure of COVID-19 (RUXO-SIM-20). In this project we propose the combined use of one of these drugs, ruxolitinib with simvastatin, looking for a synergistic effect in the inhibition of viral entry and in the anti-inflammatory effect.
- Colchicine versus ruxolitinib and secukinumab in open prospective randomized trial (COLORIT). Patients with mild and severe coronavirus disease 2019 (COVID 19) will be randomized 3:1:1:3 into four groups: colchicine, ruxolitinib, secukinumab, and control groups.
- Study of the efficacy and safety of ruxolitinib to treat COVID-19 pneumonia.
Pacritinib trials
Tofacitinib trials
- Tofacitinib treatment for moderate COVID-19
- Tofacitinib plus hydroxychloroquine in patients with COBID-19 interstitial pneumonia
- Tofacitinib in SARS-CoV2 pneumonia
- Tofacitinib in hospitalized patients with COVID19 pneumonia. To assess the safety and efficacy of tofacitinib plus standard pharmacologic and supportive measures in treating hospitalized participants with COVID-19 pneumonia.
Upacitinib: no current trials found
Fedratinib: no current trials found