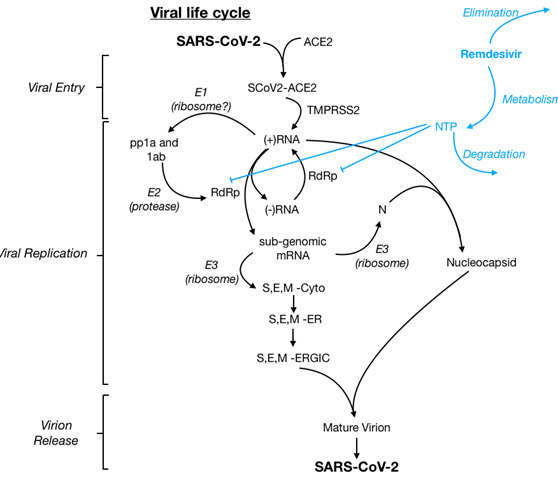
Remdesivir is a novel antiviral drug in the class of nucleotide analogs. Remdesivir is incorporated into viral RNA chains causing their premature termination. It was developed by Gilead Sciences as a treatment for viral hemorrhagic fever, though it subsequently was found to show antiviral activity against other single-stranded RNA viruses, including coronaviruses. Several trials have emerged evaluating remdesivir in hospitalized patients with COVID-19.
This medication is recommended. It is FDA approved for use in adult and pediatric patients 12 years of age and older and weighing at least 40 kilograms (about 88 pounds) for the treatment of COVID-19 requiring hospitalization. Remdesivir should only be administered in a hospital or in a healthcare setting capable of providing acute care comparable to inpatient hospital care.
Clinical Circumstances
What severity of COVID-19 would you recommend use of this medication (i.e. mild, moderate, or severe illness; outpatient vs inpatient use)?
- Inpatient use of Remdesivir is currently recommended in patients who present within < 7 days of onset of symptoms and with moderate to severe disease (defined as patients with evidence of pneumonia on chest imaging, or and oxygen saturation (SpO2) ≤94% on room air, or requiring receiving supplemental oxygen (i.e. nasal canal, oxyplus and high flow nasal cannula). The use of remdesivir within < 7 days from symptom onset in patients with moderate to severe disease may decrease progression to critical disease (i.e. mechanical ventilation). However, no clinical trial has demonstrated any mortality benefit, decreased length of hospitalization, or ICU free days with the use of Remdesivir in patients on mechanical ventilation or on ECMO. Therefore, the use of remdesivir in hospitalized patients requiring mechanical ventilation or ECMO is currently not recommended. Patients who have been started on Remdesivir prior to intubation may finish their 5-day course.
- Outpatient use of Remdesivir is currently recommended for patients with mild to moderate COVID-19 who are at high risk of progressing to severe disease (i.e. hospitalization or death). Remdesivir for the treatment of non-hospitalized COVID-19 patients at high risk for disease progression demonstrated an 87% reduction in COVID-19 related hospitalization or death at day 28 as compared to placebo. The use of Remdesivir in mild to moderate COVID-19 is recommended as a 3-day course (200 mg IV day 1, followed by 100 mg IV on days 2 and 3).
- Given the results of the PINETREE study consideration may be given for the use of Remdesivir (3 day course) in hospitalized patients with mild disease presenting with a symptom onset of 5 days and who are at high risk for disease progression.
Recommendations on restrictions for this medication:
- It is FDA approved as above in hospitalized patients. The use of Remdesivir in non-hospitalized patients is off-label.
- It should not be started in those who have already progressed to mechanical ventilation or ECMO. It can be considered in this context in consultation with Infectious Diseases Providers. Its use will be evaluated for appropriateness by the antibiotic stewardship team.
Medication Specific Considerations
Drug monitoring:
- If AST/ALT increases >5X ULN then hold Remdesivir
- If bradycardia occurs, observe. Should resolve with completion of the drug. Stop Remdesivir if clinically significant bradycardia
- If GFR falls < 30 mL/min while the patient is on Remdesivir it is okay to continue.
Supply and procurement consideration:
- No. It can be ordered by any provider.
Use in special populations:
- There is minimal data regarding the use of remdesivir in patients who are <18 years old, pregnant, or immunosuppressed. For recommendations in renal and/or hepatic dysfunction, pregnancy, see Table 1
Table 1:
| Population | Dose | Duration |
|---|---|---|
| Adults who are not requiring mechanical ventilation and/or ECMO |
|
5 days |
| Adults who require mechanical ventilation and/or ECMO |
|
N/A |
| Pediatric patients ≥40 kg |
|
5 days |
| Pediatric patients 3.5 kg to <40 kg |
|
5 days |
Renal: Remdesivir is not recommended in adult and pediatric patients >28 days old with eGFR less than 30 mL/min or in full-term neonates (≥7 days to ≤28 days) with serum creatinine ≥1 mg/dL unless the potential benefit outweighs the potential risk. There is a paucity of data to guide treatment in those with renal disease.
Hepatic: Remdesivir should not be initiated in patients with ALT ≥5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop ALT ≥5 times the upper limit of normal (but may be restarted when ALT is <5 times the upper limit of normal). Remdesivir should also be discontinued in patients who develop any ALT elevation which is accompanied by signs or symptoms of liver dysfunction during treatment.
Pregnancy: Remdesivir should be used during pregnancy only if the potential benefit justifies the potential risk for the mother and the fetus. There is a paucity of data to guide that.
 = Supporting use article = Supporting use article |
 = Neutral Article = Neutral Article |
 = Contradicting use article = Contradicting use article |

     |
  |
 |
    |
        |
   |
Major peer-reviewed studies
 Meta-analysis
Meta-analysis
 Juul S, Nielsen EE, Feinberg J, et al. Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). Plos Medicine. 2020 Sept 17. doi: 10.1371/journal.pmed.1003293.
Juul S, Nielsen EE, Feinberg J, et al. Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). Plos Medicine. 2020 Sept 17. doi: 10.1371/journal.pmed.1003293. Ansems K, Grundeis F, Dahms K, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8:CD014962. Epub 2021 Aug 5.
Ansems K, Grundeis F, Dahms K, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8:CD014962. Epub 2021 Aug 5.
 RCT SARS CoV-2
RCT SARS CoV-2
 Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020.
Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med. 2020 Oct 8 NEJMoa2007764. doi: 10.1056/NEJMoa2007764. Online ahead of print.
Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med. 2020 Oct 8 NEJMoa2007764. doi: 10.1056/NEJMoa2007764. Online ahead of print. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19 [published online ahead of print, 2020 May 27]. N Engl J Med. 2020;10.1056/NEJMoa2015301.
Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19 [published online ahead of print, 2020 May 27]. N Engl J Med. 2020;10.1056/NEJMoa2015301. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020 Sep 15;324(11):1048-1057. doi: 10.1001/jama.2020.16349.
Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020 Sep 15;324(11):1048-1057. doi: 10.1001/jama.2020.16349.- Gottlieb RL, Vaca CE, Pardes R, et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. NEJM. Dec 22, 2021.DOI: 10.1056/NEJMoa2116846.
 WHO Solidarity Trial Consortium, Pan H, Peto R, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384(6):497. Epub 2020 Dec 2.
WHO Solidarity Trial Consortium, Pan H, Peto R, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384(6):497. Epub 2020 Dec 2. Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3 randomised, controlled, open-label trial. Lancet Infect Dis. 2021 Sep. https://doi.org/10.1016/S1473-3099(21)00485-0
Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3 randomised, controlled, open-label trial. Lancet Infect Dis. 2021 Sep. https://doi.org/10.1016/S1473-3099(21)00485-0
 Observational SARS CoV-2
Observational SARS CoV-2
 Grein J, Ohmagari N, Shin D, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. NEJM. 2020.
Grein J, Ohmagari N, Shin D, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. NEJM. 2020. Humeniuk R , Mathias A, Cao H et al. Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID-19, in Healthy Subjects. Clin Transl Sci. 2020 Sep;13(5):896-906. doi: 10.1111/cts.12840. Epub 2020 Aug 5.
Humeniuk R , Mathias A, Cao H et al. Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID-19, in Healthy Subjects. Clin Transl Sci. 2020 Sep;13(5):896-906. doi: 10.1111/cts.12840. Epub 2020 Aug 5. Olender SA, Perez KK, Go AS, et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care. Clinical Infectious Diseases, ciaa1041, 24 July 2020 https://doi.org/10.1093/cid/ciaa1041
Olender SA, Perez KK, Go AS, et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care. Clinical Infectious Diseases, ciaa1041, 24 July 2020 https://doi.org/10.1093/cid/ciaa1041
 In vivo MERS-CoV
In vivo MERS-CoV
 de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 2020;13:1922083117.
de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 2020;13:1922083117.
 In vitro SARS CoV-1/2 and MERS-CoV
In vitro SARS CoV-1/2 and MERS-CoV
 Agostini ML, Andres EL, Sims AC, et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9(2):e00221-18. Published 2018 Mar 6.
Agostini ML, Andres EL, Sims AC, et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9(2):e00221-18. Published 2018 Mar 6.
- In vitro study (SARS-CoV-1)
 Gordon CJ, Tchesnokov EP, Feng JY, et al. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem 2020;24:013056.
Gordon CJ, Tchesnokov EP, Feng JY, et al. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem 2020;24:013056.
- In vitro study (MERS)
 Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017;9.
Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017;9.
- In vitro study (SARS-CoV-1, MERS)
 Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro: Cell Res. Mar;30(3):269-271. Epub 2020 Feb 4.; 2020.
Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro: Cell Res. Mar;30(3):269-271. Epub 2020 Feb 4.; 2020.
- In vitro study (SARS-CoV-2)
- In vitro study (SARS-CoV-2)
Extended Review
- Remdesivir is a novel antiviral drug in the class of nucleotide analogs. Remdesivir incorporates into viral RNA chains causing their premature termination. It was developed by Gilead Sciences as a treatment for viral hemorrhagic fever, though it subsequently was found to show antiviral activity against other single-stranded RNA viruses, including coronaviruses.
- Preliminary reports using remdesivir were very promising and it was used throughout 2018-2019 until RCT data was published in August 2019
- RCTs identified that remdesivir was significantly less efficacious than mAb114 and REGN-EB3 and its use was discontinued.
- SARS-CoV-1: Remdesivir has never been tested in SARS-CoV-1
- MERS-CoV
- In vivo primates
- De Wit et al. identified significantly reduced viral load and lung histology score in primates infected with the MERS-CoV virus treated with therapeutic remdesivir. It was more effective in this model as a prophylactic medication
- In vivo primates
- COVID-19
- Clinical studies
- Ader et al evaluated the clinical efficacy of remdesivir plus the standard of care compared with standard of care alone in hospitalized patients.
- Randomized control trial (open-label,adaptive, multi-center) conducted at 48 sites. Received standard of care alone or standard of care with one of the following: remdesivir, lopinavir-ritonavir, lopinavir-ritonavir with interferon beta-1a or hydroxychloroquine. This summary focuses on the outcomes with standard of care with remdesivir as compared to standard of care alone.
- Enrollment Mar 22, 2020-January 21, 2021
- Included adult patients (age >18 years old) who were hospitalized with confirmed SARS-CoV-2 infection and any duration of illness was included with:
- Evidence of pneumonia with hypoxia
- Or requiring supplemental O2
- Exclusion were elevated liver enzymes, severe CKD or contraindication to a study medication
- Outcomes:
- Primary: Clinical status (as defined by WHO seven-point ordinal scale) at day 15.
- Overall, the differences across the seven-point ordinal scale between treatment groups at day 15 was not significant (odds ratio 0·98 [95% CI 0·77–1·25]; p=0·85)
- The difference between treatment groups at day 29 across the seven point ordinal scale showed no difference (odds ratio 1.11 [95% CI 0.87-1.42]; p= 0.39)
- In a subset analysis remdesivir did delay the need for new mechanical ventilation or ECMO or death, however, as part of the secondary outcome analysis this effect was not observed and did not reduce mortality at day 28.
- Secondary: safety as described by serious adverse events
- No significant difference was observed between treatment groups (remdesivir, 135 [33%] of 406 vs control, 130 [31%] of 418; p=0·48).
- Viral kinetics evaluation: As found in other studies, remdesivir had no effect on SARS-CoV2 viral kinetics.
- Grein et al described outcomes from compassionate use of remdesivir in a group of 53 patients with no control group.
- The median age was 64 years (interquartile range, 48 to 71), and at baseline, 64% of the patients were receiving invasive ventilation, including 4 people (8%) on ECMO.
- Over a median of 18 days after receiving the first dose of remdesivir, 68% showed an improvement in their need for oxygen support, and 15% showed worsening.
- The mortality rate after 28 days was 13% overall and 18% within the group which required invasive ventilation prior to enrollment
- 60% of the patients reported any adverse events during follow-up and 23% had serious adverse events, although it could not be determined whether the cause of these events was the study medication or the underlying disease process.
- Ader et al evaluated the clinical efficacy of remdesivir plus the standard of care compared with standard of care alone in hospitalized patients.
- Wang et al described outcomes from a randomized, controlled, double-blinded, multi-center trial in China
- Included 237 hospitalized adults with laboratory-confirmed SARS-CoV-2 infection, with an interval from symptom onset to enrollment of 12 days or less, oxygen saturation of 94% or less on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen of 300 mm Hg or less, and radiologically confirmed pneumonia
- Randomized 2:1 to IV remdesivir (200 mg on day 1, then 100 mg daily on days 2–10) or placebo. Use of lopinavir–ritonavir, interferons, and corticosteroids was allowed
- Primary endpoint: time to clinical improvement up to day 28, defined as days from randomisation to the point of a decline of two levels on a six-point ordinal scale of clinical status (from 1=discharged to 6=death) or discharged alive from hospital, whichever came first
- n=158 received remdesivir and 79 received placebo; study was terminated prior to meeting power based on the termination criteria prespecified in the protocol
- No difference in primary outcome: hazard ratio 1·23 [95% CI 0·87–1·75]
- Remdesivir was associated with a numerically faster time to clinical improvement among patients with symptom duration of 10 days or less (hazard ratio 1·52 [0·95–2·43]), but this was not statistically significant
- AEs were reported in 66% of remdesivir recipients vs 64% of placebo recipients. The AEs reported in multiple patients from remdesivir > placebo were: rash, thrombocytopenia, increased T bili, increased neutrophils, nausea
- The Adaptive COVID-19 Treatment Trial (ACTT) was a double-blind, randomized, placebo-controlled trial with the primary endpoint of time to recovery (defined as hospital discharge or hospitalization for infection control purposes only).
- This study enrolled 1,063 patients with COVID-19 and evidence of lower respiratory tract infection. The participants in both the remdesivir and placebo groups were similar at baseline.
- Patients treated with remdesivir had a 31% faster median time to recovery than placebo (11 days versus 15 days, RR for recovery 1.32, 95% CI 1.12-1.55, p<0.001).
- The subgroup of patients receiving mechanical ventilation or ECMO at enrollment had a rate ratio for recovery of 0.95 (95% CI 0.64-1.42), thus the investigators did not observe faster recovery compared to placebo in this group.
- Mortality was numerically lower in the remdesivir group but was not statistically significant (8.0% versus 11.6%, p=0.059)
- This study was stopped early due to the finding of superiority of remdesivir for the primary outcome and the result were published prior to all participants completing follow-up.
- Goldman et al describes a randomized, open-label, phase 3 trial completed in hospitalized patients with COVID-19, radiologic evidence of pneumonia, and an oxygen saturation of 94% or less on ambient air.
- N=397 patients were randomized to receive remdesivir for either 5 days or 10 days. The authors estimated that a sample size of 400 patients would provide at least 85% power to detect clinically significant improvement.
- The primary outcome was clinical improvement by at last 2 points on an ordinal scale.
- After adjusting for baseline clinical status, the primary outcome was not different between the 5-day and 10-day groups (P=0.14).
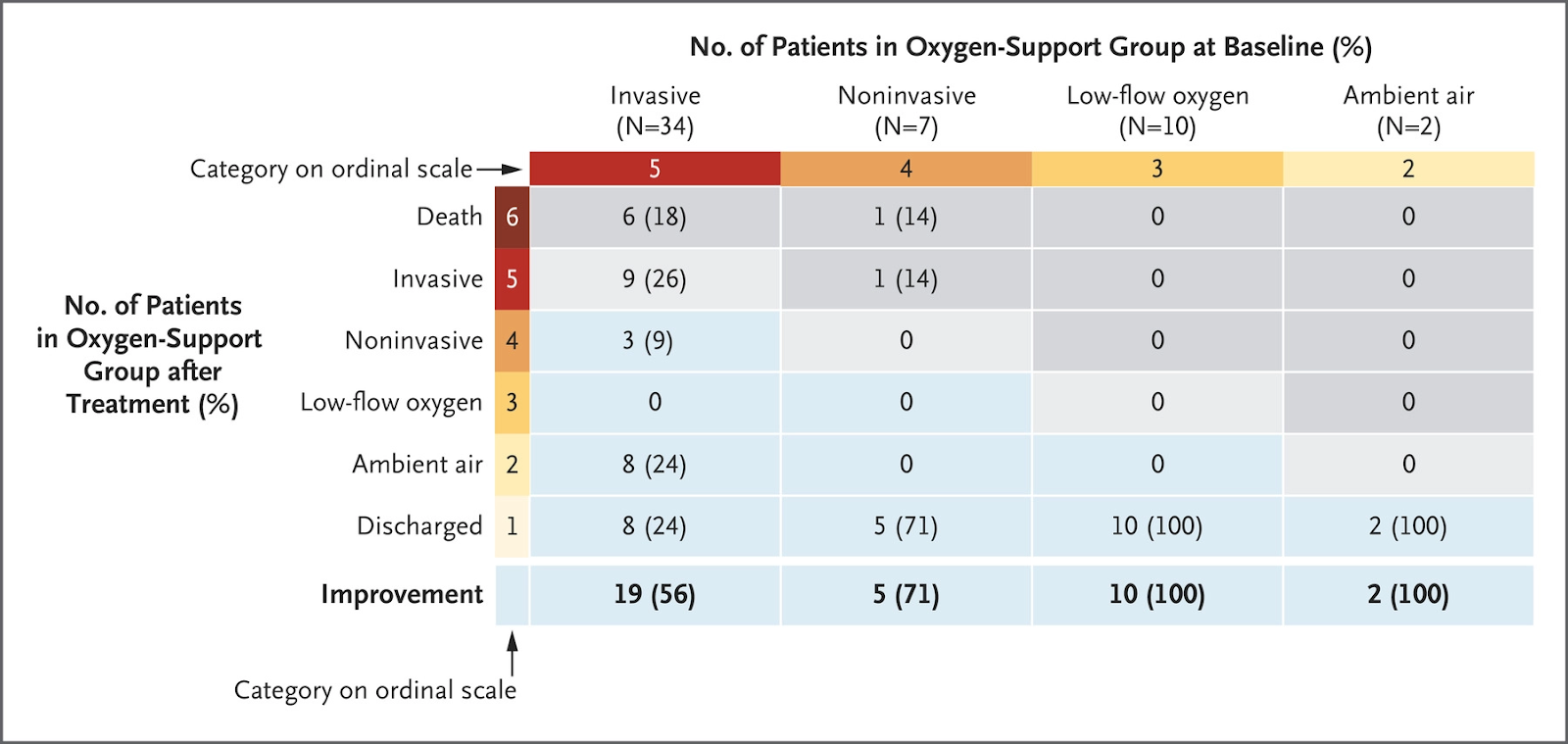
- Spinner et al. describes clinical outcomes on day 11 from a randomized clinical trial comparing 5-day course, 10-day course of remdesivir vs. standard of care.
- Patient population: Moderate COVID-19 pneumonia (any radiographic evidence of pulmonary infiltrates and oxygen saturation above 94% on room air)
- 596 patients randomized (584 began the trial) in a 1:1:1 ratio between 5 day, 10 day, standard of care courses. The median age was 57 (interquartile range 46-66).
- Patients received 200mg IV on day one with 100mg IV daily for the remainder of course. Treatment discontinued if ALT rose >3x upper limit of normal, total bilirubin rose >2x upper limit of normal or CrCl dropped <30mL/mn.
- Patients evaluated daily from day 1 until day 14 or discharged. A 7 point ordinal scale (1= Death, 7=Discharge) based on necessary treatments or outcomes was used.
- 1 - Death
- 2 - Hospitalized, requiring mechanical ventilation or ECMO
- 3 - Hospitalized, requiring non-invasive ventilation/high-flow oxygen devices
- 4 - Hospitalized, requiring low-flow supplemental oxygen
- 5 - Hospitalized, not requiring supplemental oxygen but requiring ongoing medical care related or not to COVID-19
- 6 - Hospitalized, not requiring supplemental oxygen or ongoing medical care
- 7 - Not hospitalized
- Primary efficacy endpoint: Distribution of clinical status on day 11 evaluation
- Exploratory Efficacy Endpoints:
- Time to 2 point or greater clinical improvement
- Time to 1 point or greater improvement in clinical status
- Time to recovery
- Time to modified recovery
- Time to discontinuation of oxygen support
- Calculated that 600 patients (200 in each group) would provide >85% power to detect odds ratio of 1.8.
- 5-day remdesivir group: Of 191 patients, 145 (76%) completed treatment duration
- 35 (18%) discharged
- 5 (3%) withdrew consent
- 4 (2%) had adverse events
- 10-day remdesivir group: Of 193 patients 73 (38%) completed assigned treatment duration
- Median number of doses was 6
- 98 (51%) discharged
- 8 (4%) adverse events
- 6 (3%) withdrawal of consent
- On day 11 patients in the 5-day course had statistically significant higher odds of having better clinical status when measured on the 7 point ordinal scale when compared to standard of care.
- Odds ratio: 1.65, 95% CI: 1.09-2.48, P=0.02
- Difference in clinical status on day 11 between 10-day course group and standard of care was not statistically significant (P=0.18)
- On day 14 clinical status of both 5 and 10 day courses of remdesivir groups were significantly different than that of standard of care (P= 0.03, both groups)
- On day 28 Clinical status remained significantly different in the 10-day remdesivir treatment group (P=0.03)
- Among patients with moderate COVID 19 patients randomized to a 10-day course of remdesivir did not have a statistically significant clinical improvement on day 11 compared to standard of care. While patients randomized to a 5 day course of remdesivir had a statistically significant difference in clinical status compared to standard of care. This difference is of uncertain clinical importance.
Current Clinical Trials
- Adaptive COVID-19 Treatment Trial (ACTT)
- ACTT-1 enrollment goal reached and enrollment closed 4/19/2020.
Preliminary results from ACTT-1 indicate that patients who received remdesivir had a 31% faster time to recovery than those who received placebo (p<0.001). Median time to recovery was 11 days for patients treated with remdesivir compared with 15 days for those who received placebo. Results suggest a survival benefit, mortality rate of 8.0% for the group receiving remdesivir versus 11.6% for the placebo group (p=0.059). https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19
Final results of ACTT-1 are now published in the NEJM, and the final results are in line with the preliminary results. Those study participants who received remdesivir had a median time to recovery of 10 days as compared with 15 days among those who received placebo (rate recovery ratio 1.29; 95% CI, 1.12 to 1.49; P<0.001, by a log-rank test). Mortality was 11.4% with remdesivir and 15.2% with placebo by day 29 (hazard ratio,0.73; 95% CI,0.52 to 1.03). In summary the final data from ACTT-1 show that remdesivir was superior to placebo in shortening time to recovery in adults who were hospitalized with COVID-19 and had evidence of lower respiratory tract infection.
- ACTT-1 enrollment goal reached and enrollment closed 4/19/2020.
-
Early Remdesivir to Prevent Progression to Severe COVID-19 in outpatients.
- Randomized, double-blinded, placebo-controlled trial of non-hospitalized patients with COVID-19, with symptoms within 7 days, and with at least one risk factor for disease progression.
- Study population: 279 patients received Remdesivir, 283 patients received placebo.
- Primary end-point - COVID-19 related hospitalization or death by day 28 was 2(0.7%) in the Remdesivir arm and 15(5.3%) in the placebo group (p=0.008).
- Conclusion: Among non-hospitalized patients with mild to moderate COVID-19 who presented within 7 days of symptom onset who received Remdesivir had a 87% lower risk of hospitalization or death than those who had received placebo.